Pfizer vaccine leads to 'significantly lower' case rates in nursing homes, Yale study finds - Yale Daily News

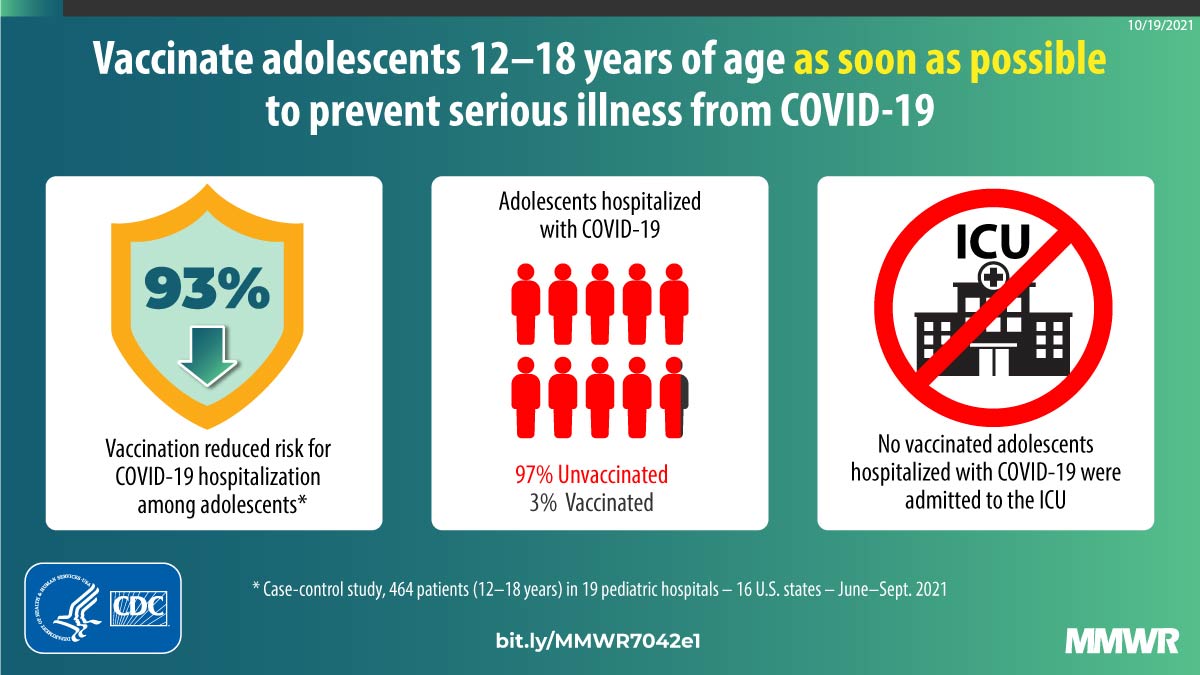
Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA Vaccination Against Multisystem Inflammatory Syndrome in Children Among Persons Aged 12–18 Years — United States, July–December 2021 | MMWR

pfizer vaccine: Pfizer wants vaccine disputes only in US Courts apart from indemnity waiver in case of adverse effects - The Economic Times
Pfizer loses appeal in patent case concerning Lyrica use in pain relief - The Pharmaceutical Journal