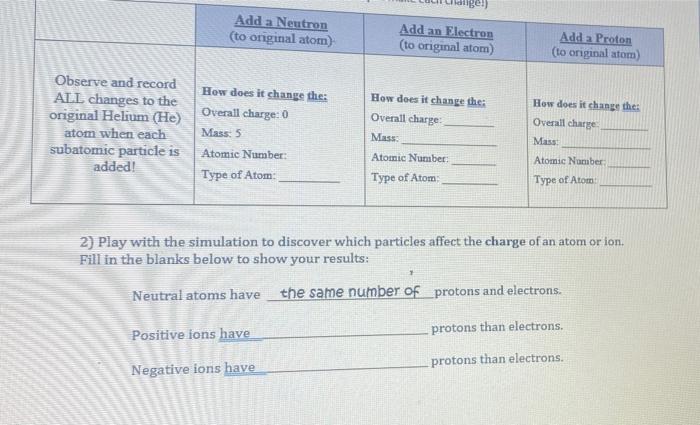
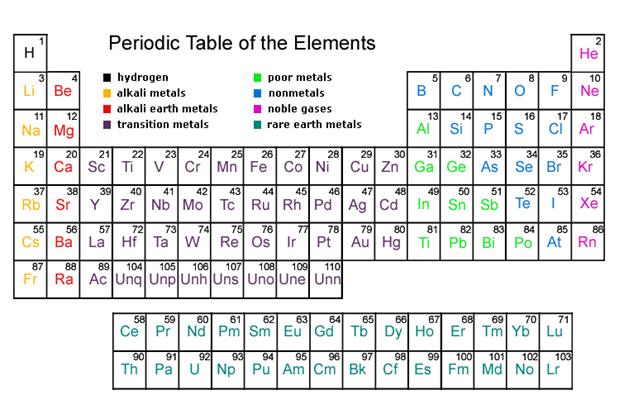
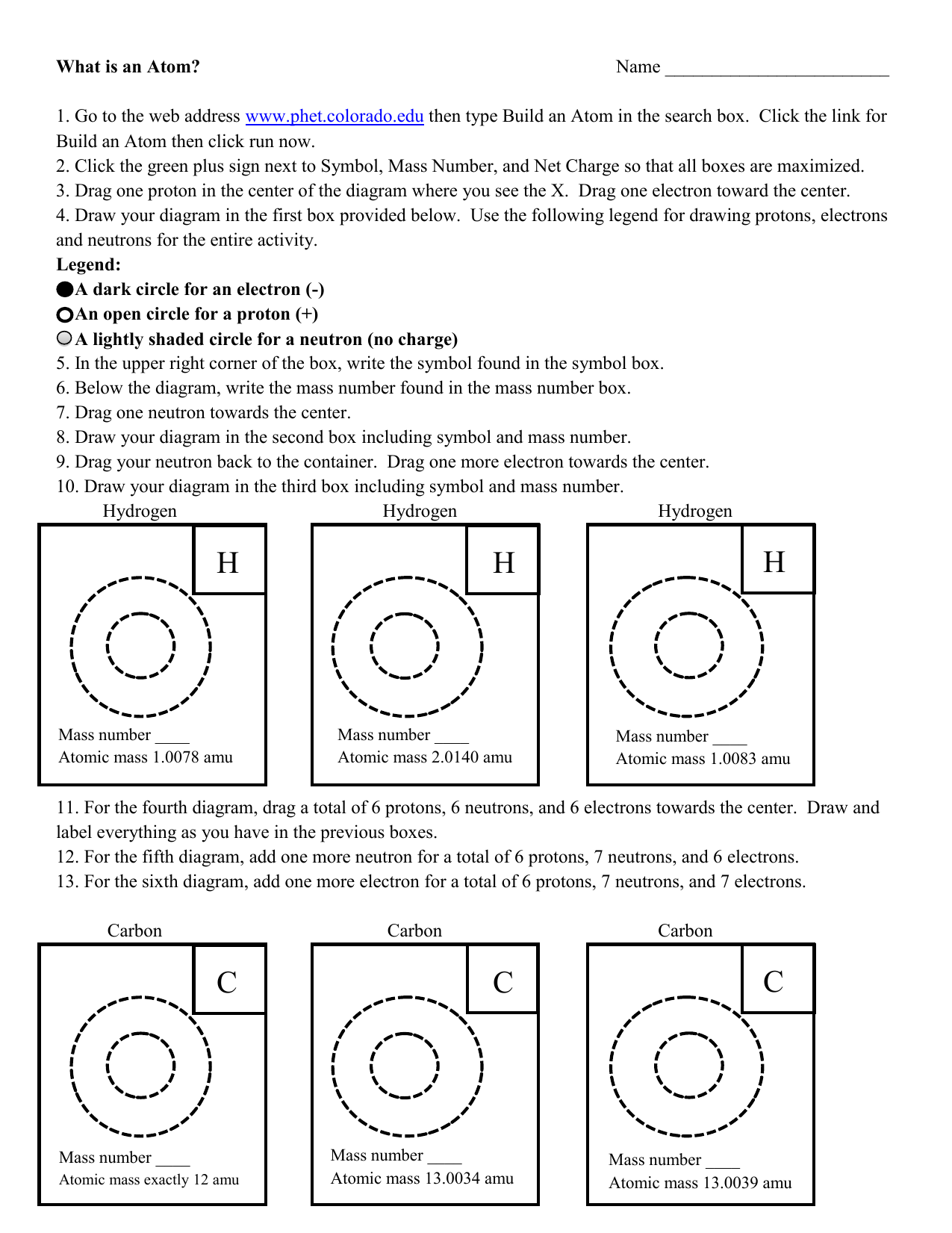
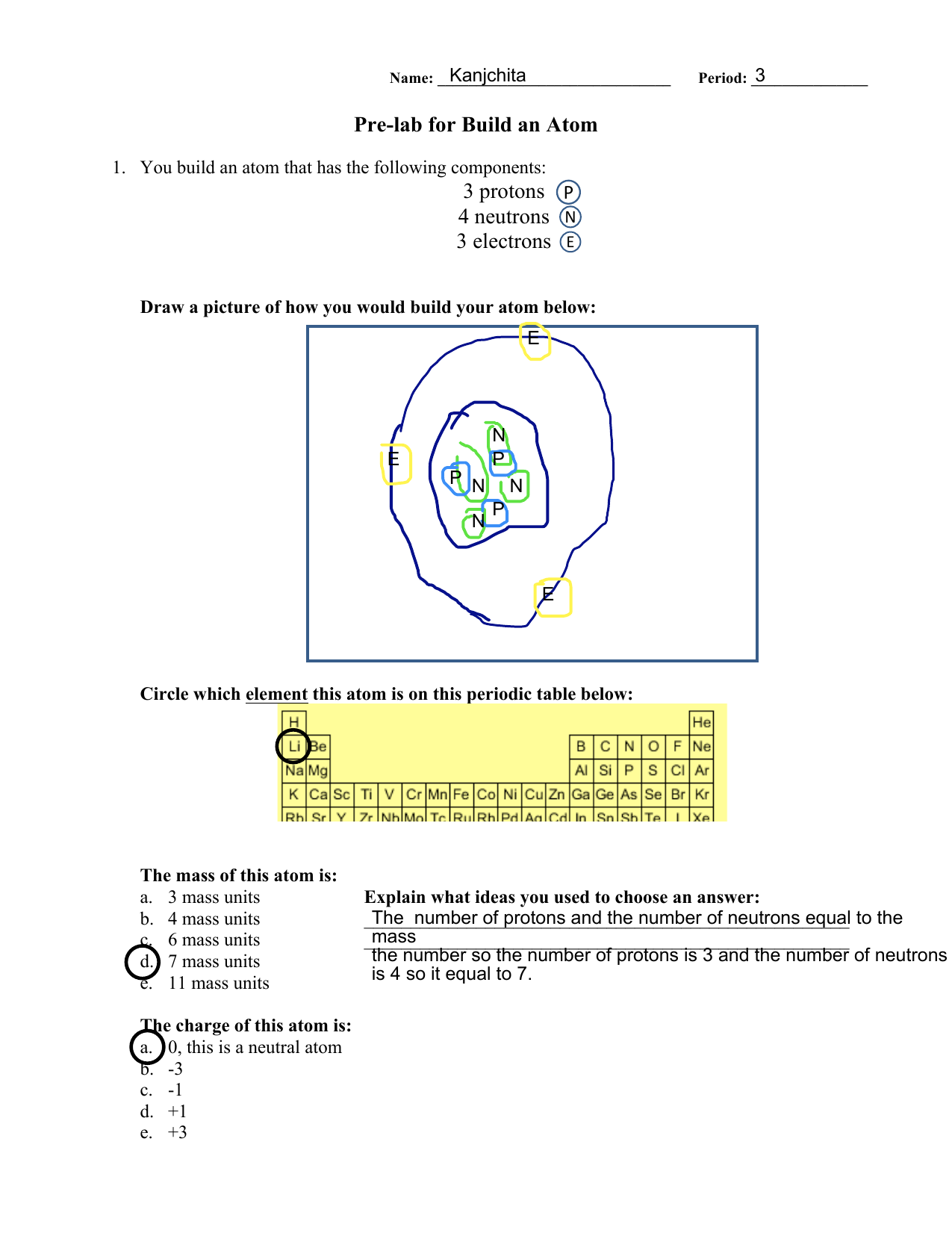
Chapter 2 Chemistry Lecture Worksheets 2019.docx - Atom Building Activity You will use a simulation activity from the University of Colorado's PhET | Course Hero
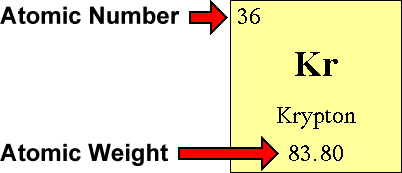
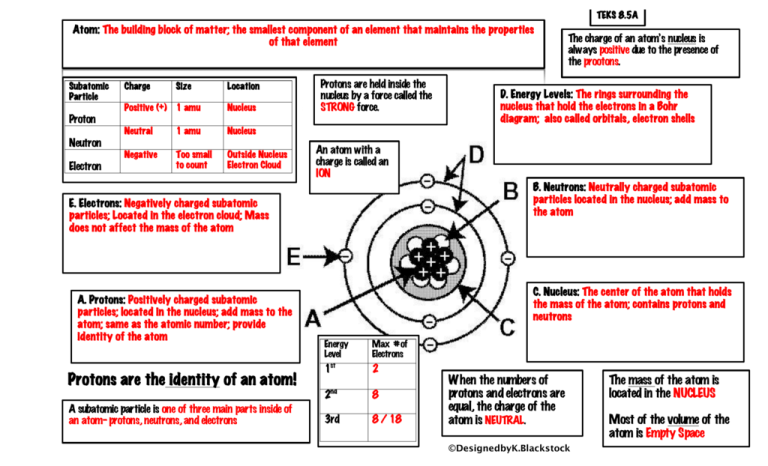
Questions and Answers - How do I find the number of protons, electrons and neutrons that are in an atom of an element?

SOLVED:Consider an atom of 10 $\mathrm{B}$ . (a) How many protons, neutrons, and electrons does this atom contain? (b) What is the symbol of the atom obtained by adding one proton to $^{

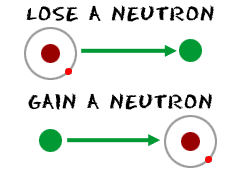
Adding neutrons to synthetic atoms drastically alters shape of their nuclei, affects their stability


/GettyImages-1017116892-917f9457f2bc4e4cbca2827b9d0a8966.jpg)